When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how does the FDA make sure it actually does? The answer lies in bioequivalence-a scientific standard that bridges the gap between brand-name drugs and their cheaper copies. It’s not about matching ingredients exactly. It’s about proving that your body absorbs and uses the generic drug the same way it does the original.
What Bioequivalence Really Means
Bioequivalence isn’t about the amount of active ingredient in the pill. That’s already controlled by pharmaceutical equivalence: same drug, same dose, same form. Bioequivalence goes deeper. It asks: Does the drug enter your bloodstream at the same speed and in the same amount as the brand-name version?
The FDA defines bioequivalence as the absence of a significant difference in the rate and extent to which the active ingredient becomes available at the site of action. In plain terms: if you take a generic version of a blood pressure pill, your body should see the same levels of the drug over time as if you took the brand-name one. No more, no less.
This standard was created by the Hatch-Waxman Act of 1984. Before that, generic manufacturers had to repeat expensive clinical trials just to prove their drug worked. The law changed that. Instead, they could rely on the original drug’s safety data-if they could prove bioequivalence. That’s how we got affordable generics without sacrificing safety.
How the FDA Tests for Bioequivalence
The gold standard is a clinical study in healthy volunteers. These aren’t patients with the disease. They’re people with no major health issues, typically between 24 and 36 individuals. They’re given the brand-name drug and the generic version in a randomized, crossover design-meaning half take the brand first, then the generic; the other half do the reverse. This controls for individual differences in metabolism.
Researchers then measure two key things:
- Cmax: The highest concentration of the drug in the blood after taking it. This tells you how fast the drug gets absorbed.
- AUC: The total exposure over time-how much of the drug your body absorbs overall. This is calculated from the area under the concentration-time curve.
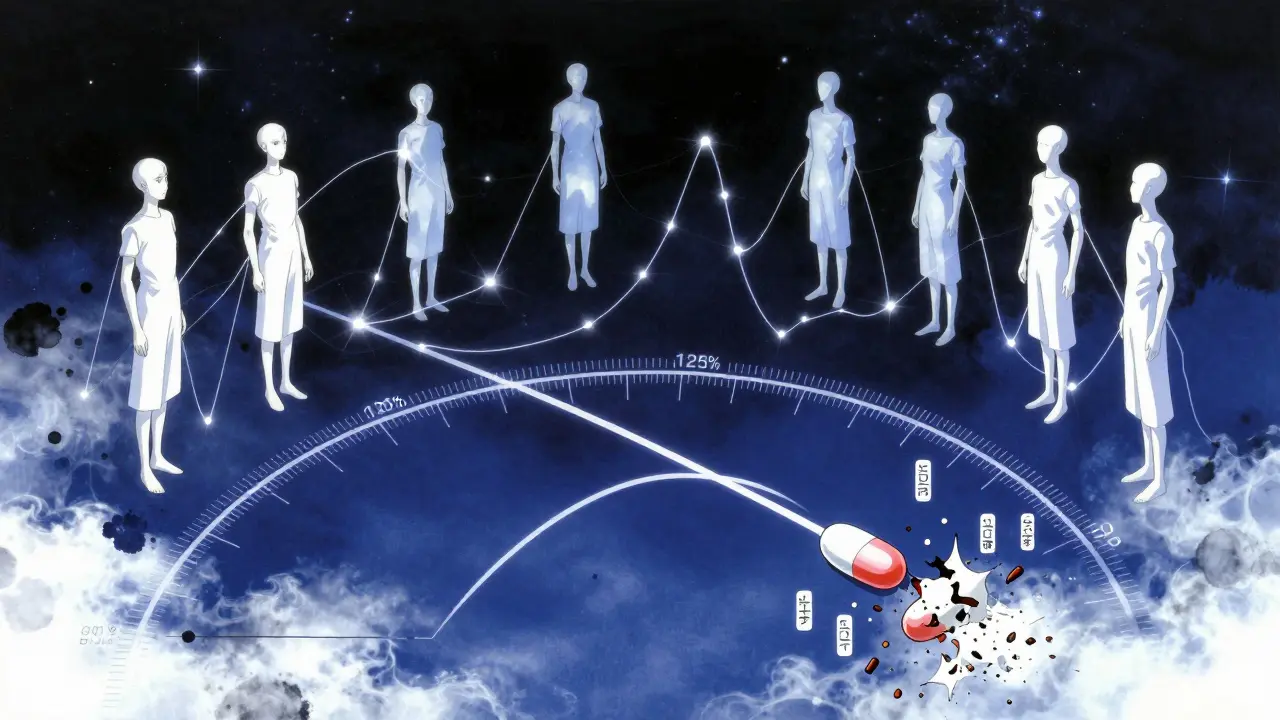
For the generic to be approved, the 90% confidence interval (CI) of the ratio between the generic and brand-name drug must fall between 80% and 125% for both Cmax and AUC. That means if the brand’s average AUC is 100 units, the generic’s must be between 80 and 125 units. But here’s the catch: it’s not just the average. The entire range of likely values-your 90% CI-must stay within those bounds.
For example: If the generic’s average AUC is 93, but the 90% CI runs from 84 to 110, it passes. All values are between 80 and 125. But if the average is 116% and the CI stretches from 103% to 130%, it fails-even though the average looks close. The upper end breaks the limit.
This isn’t arbitrary. The 80-125% range comes from statistical analysis of clinical data. Experts agree that a 20% difference in absorption is unlikely to affect how a drug works for most conditions. But for some drugs, like those with a narrow therapeutic index (think warfarin, lithium, or certain epilepsy meds), even small differences can matter. The FDA still uses the same range, but these drugs get extra scrutiny during review.
Myth Busting: The 80-125% Misunderstanding
One of the most persistent myths is that generic drugs can contain 80% to 125% of the active ingredient. That’s wrong. The 80-125% range applies to how your body handles the drug-not how much is in the tablet.
Every generic pill must contain the exact same amount of active ingredient as the brand. If the brand has 50 mg, the generic has 50 mg. The FDA requires this. The variability comes from how fast your body absorbs it, how your gut processes it, or how the tablet breaks down. Two pills with identical ingredients can behave differently in your body because of fillers, coatings, or manufacturing methods.
That’s why bioequivalence studies are so important. They’re not checking the pill’s label-they’re watching what happens inside you.
When In Vitro Testing Is Enough
Not all drugs need to be tested in people. For medications that work locally-like inhalers, eye drops, or topical creams-the FDA may allow in vitro testing instead. That means testing how the drug dissolves or spreads in a lab setting, not in human blood.
For example, a generic asthma inhaler doesn’t need to be tested in volunteers if it matches the brand’s particle size, spray pattern, and dissolution rate in controlled lab conditions. The FDA has specific product-specific guidances for over 2,000 drugs that outline exactly what’s needed. These are public and updated regularly.
But for pills, injections, or any drug meant to enter your bloodstream, in vivo testing is required. That’s where the Cmax and AUC data come from.
What Happens Behind the Scenes
Applying for FDA approval of a generic drug means submitting an Abbreviated New Drug Application, or ANDA. The process takes about 10 to 12 months on average. About 65% of first-time applications get approved without needing major changes.
But many don’t make it the first time. Common reasons for rejection? Poor dissolution profiles, inconsistent manufacturing, or bioequivalence data that doesn’t meet the 80-125% rule. Sometimes, a small change in the tablet’s coating or the way it’s pressed can throw off absorption. Manufacturers often have to tweak the formula multiple times before the FDA says yes.
Since 2021, the FDA requires companies to submit all bioequivalence studies they’ve done-not just the successful ones. This transparency helps the agency spot patterns. If a company ran five studies and only submitted the one that worked, it raises red flags. Now, they have to show the full picture.

Why This Matters for Patients
Generic drugs make up 90% of prescriptions in the U.S. but cost only about 20% of what brand-name drugs do. Over the past decade, they saved the healthcare system nearly $1.7 trillion. That’s not just a number-it means millions of people can afford their meds.
And the science backs it up. The FDA doesn’t approve a generic unless it’s proven to work the same way. Studies have shown that patients switching from brand to generic experience no increase in side effects or treatment failure for the vast majority of drugs.
Some doctors still hesitate to prescribe generics, especially for complex conditions. But that’s often based on outdated beliefs. The data doesn’t support it. The FDA’s standards are strict, transparent, and grounded in decades of evidence.
What’s Next for Bioequivalence
The FDA is exploring new ways to make testing faster and more efficient. For complex drugs like biologics, inhalers, or topical gels, traditional bioequivalence studies are hard to design. So the agency is turning to modeling and simulation-using computer programs to predict how a drug will behave in the body based on its physical properties.
These methods could eventually reduce the need for human trials for some products. But for now, the 80-125% rule remains the gold standard. It’s simple, proven, and effective.
As more complex generics enter the market, the focus is shifting toward ensuring consistency across batches. A drug that works today should work the same way six months from now. That’s why manufacturing controls are now just as important as the bioequivalence data itself.
Do generic drugs have the same active ingredient as brand-name drugs?
Yes. The FDA requires that generic drugs contain the exact same active ingredient, in the same strength and dosage form, as the brand-name version. The difference isn’t in what’s in the pill-it’s in how it’s made. Fillers, coatings, and manufacturing processes can vary, which is why bioequivalence testing is needed to confirm your body absorbs it the same way.
Can a generic drug be less effective than the brand-name version?
No-not if it’s FDA-approved. The 80-125% bioequivalence range ensures that any difference in absorption is too small to affect how the drug works. Over 90% of prescriptions in the U.S. are filled with generics, and decades of real-world use show no increase in treatment failure or side effects compared to brand-name drugs.
Why do some people say generics don’t work as well?
Sometimes, it’s a placebo effect-or confusion between different generic versions. A person might switch from one generic to another, notice a change, and blame the generic. But if both are FDA-approved, they’re both bioequivalent to the brand. The real issue might be switching between different manufacturers’ versions, which can have slightly different inactive ingredients that affect how the pill feels or tastes, not how it works.
Are there any drugs where bioequivalence doesn’t apply?
Yes. For drugs that act locally-like nasal sprays, eye drops, or topical creams-the FDA allows in vitro testing instead of human studies. Also, complex drugs like biologics (e.g., insulin or monoclonal antibodies) don’t follow the same rules. Their approval paths are different because they’re harder to replicate exactly.
How long does it take for the FDA to approve a generic drug?
The average review time for an ANDA is 10 to 12 months. About 65% of applications are approved on the first try. The rest usually need more data, often on dissolution or bioequivalence. Delays often happen when the manufacturer’s formulation doesn’t match the brand’s absorption profile, requiring reformulation.

